Minimally invasive medical devices and their applications have accelerated an array of medical procedures by minimizing incisions, shortening or eliminating hospital stays, reducing recovery times, and improving surgical and diagnostic results.
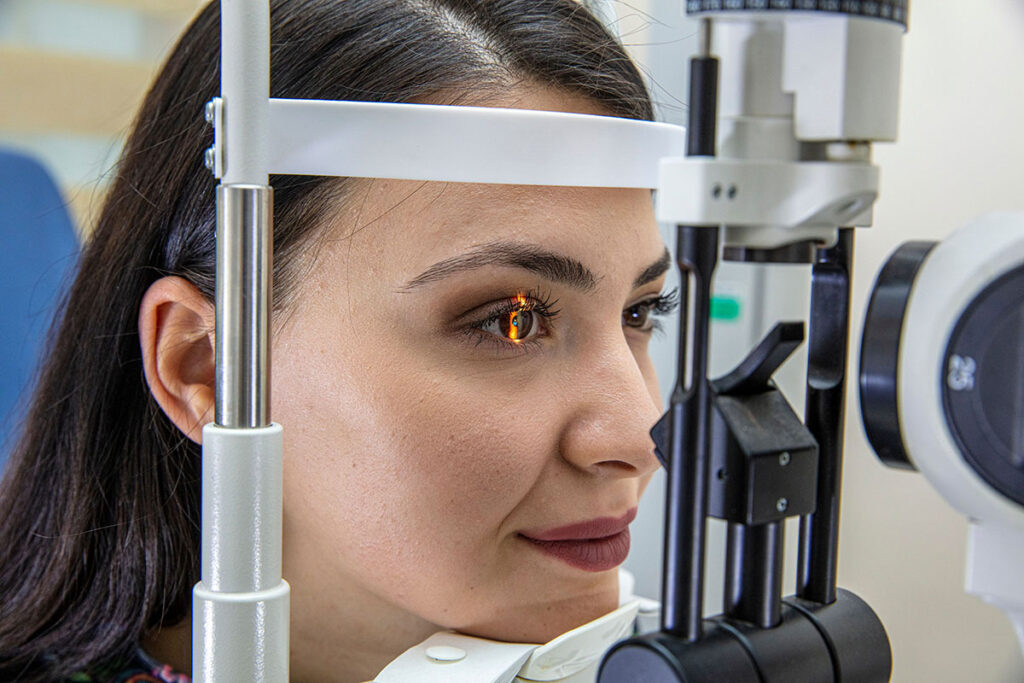
Medical devices including: surgical robotics, spinal, hip & knee retractors, surgical tools & instruments, ophthalmology laser instruments, and endoscopes coupled with advances with fiber optic laser and lighting technology, continue to make even less minimally invasive procedures possible. Exciting new surgical procedures include, but are not limited to, the ability to transform the treatment of mitral regurgitation, and newly developed ophthalmology laser instruments used for glaucoma & retinal procedures, all using custom fiber optic probes.
As a medical device project designer you have a concept in mind for a product offering, and are faced with the challenge of designing an unique laser or lighting illumination solution to meet an application requirement? Then we welcome you to speak with our design team at Innofiber.
1) Partnership. Make sure your medical design fiber optics vendor has a solid understanding of medical devices and harmonized standards.
Consider choosing a partner with extensive medical fiber optic design experience, and a history of producing results for medical device manufacturers.
Finding a fiber optic design partner with detailed understanding of mechanical, and optical engineering project applications is critical. A partner that has valuable design experience and understanding of application needs saves time and money during the product development cycle. This includes employing the proper materials and process controls, with consideration of product packaging and labeling during product development to meet stringent regulatory, harmonized standards, CE marking, and validation requirements.
For example, our Engineering Manager, Mark Stevens, explains that “partnering early with Innofiber in the concept phase allows for a fiber optic solution to be designed concurrent with the device under development. This pro-active approach allows for more design flexibility while elegantly fully integrating a laser or illumination cable design solution”.
Our project focused engineering group includes cross functional engineers, who have engineering knowledge encompassing multiple talented disciplines, including: opto-mechanical, chemical, packaging, and industrial engineering. Most importantly, with an assortment of innovative IP product solutions developed for the medical, laser, analytical instrumentation, and industrial segments, Innofiber’s novel fiber optic cable designs are developed for manufacturability.
UNIQUE CUSTOM FIBER OPTIC SOLUTION + MANUFACTURABILITY = COST COMPETITIVE PRODUCT
2) Evaluate Project Engineering Managers (PM)
Along with understanding the device under development, look for a medical design fiber optic cable supplier that has creative design solution project managers.
What’s involved in choosing an effective project manager to partner with?
Great project managers help and assist their client’s program by offering multiple design solutions that offer the client options from which to consider. They have a unique perspective of the client’s design goals and objectives, facilitating communication and ensure critical designs that are available to consider as the product design cycle evolves. Most importantly, they help the project run smoothly by looking ahead to potential barriers and regulatory harmonized standard requirements such as packaging and product labeling. These activities result in a true in-process partnership between the client & Innofiber’s OEM project design team, dedicated to its client’s success.
These managers are skilled communicators, they listen, understand the client’s technical design needs and wants, the project’s time constraints, quality requirements, & budget. As active listeners, the PM identifies the key points and takes these into consideration during the fiber optic product solution. There may be multiple approaches to a medical fiber optic product design, but our project managers are skilled in communicating effectively with the client, preventing scope creep, while delivering a prototype design working in close collaboration. This collaboration, builds trust, and strengthens the partnership between the client & supplier over time.
3) Lifecycle capability under one roof. Can your medical design partner take you from design concept through manufacture, packaging, and fulfillment?
4) Time to market. Your chosen fiber optic supplier should be able to bring the project to their client in a desired time frame
In the medical arena, quality is of number one importance. The fiber optic design needs to be reliable, meet standard protocols of OQ & PQ, and be manufacturable in a production environment. All are critical parameters of a new product being brought to market. It’s challenging to predict time to market, as every project is different, and obstacles are encountered. By having a supplier who is well organized, has a tollgate process in place, is experienced in all the protocol facets for qualification and testing of non-disposable and disposable fiber optic devices that are well documented, both from the perspective of its engineered design and manufacturability, the cycle is optimized.
While price is always a consideration, product quality and on time delivery are but two additional important facets that play key roles in time to market. As obstacles occur during the cycle, it’s the supplier’s past experiences and levels of performance that minimize issues to meet a successful time to market product release.
By following these guidelines in choosing a medical device fiber optic partner, a company is more likely to get a quality product that meets both the unique needs of the device, is manufacturable, and is within the project budget scope of the program.



